Published : 2024-06-08
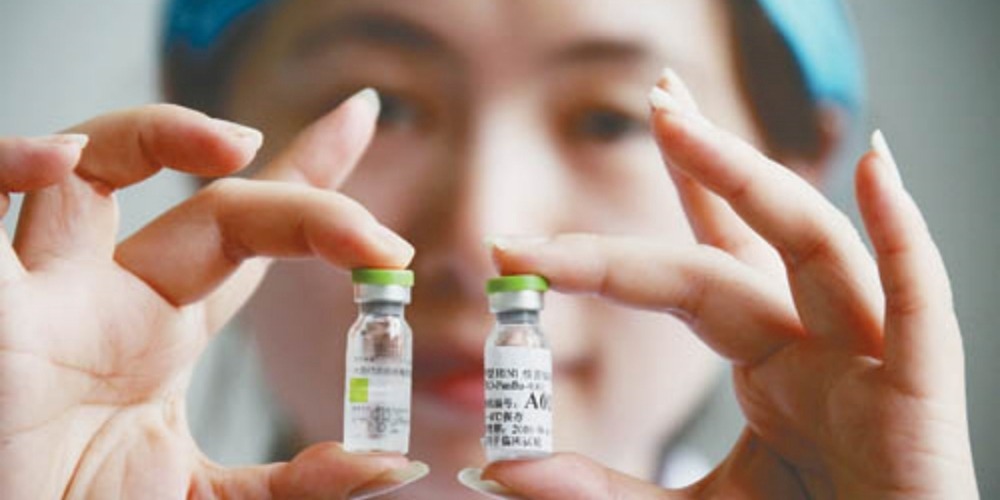
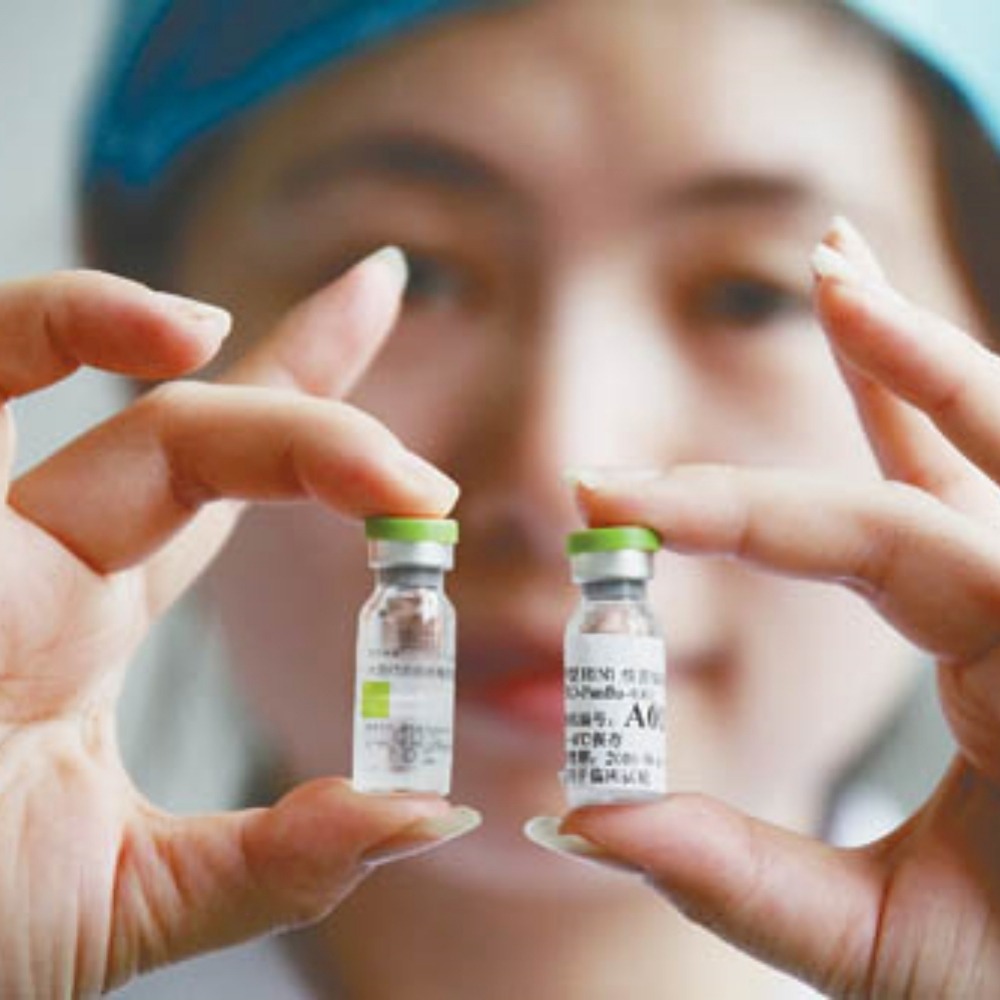
On June 8, 2009, Sinovac Biotech Ltd.(科興生物) in Beijing officially started mass production of the H1N1 influenza vaccine "Panflu"(盼爾來福).
China confirmed its first case of H1N1 influenza on May 11 of that year. The patient was a 30-year-old male who had been studying at a university in the United States and returned to China in the early morning of May 9.
As early as 2004, Sinovac Beijing started a collaboration with the Chinese Centre for Disease Control and Prevention (China CDC) and began the research on pandemic influenza prototype vaccine with the support from the Ministry of Science and Technology and the Ministry of Health.
They went on to build a brand new automated packaging line with support of the National Development and Reform Commission. Sinovac Beijing holds the unique qualification for the production of pandemic influenza vaccine in China, with a design capacity of 20 to 30 million doses a year.
However, Sinovac Beijing's production capacity could not entirely meet the demand of the state and the public.
Hence, it decided to form an alliance with several domestic seasonal influenza vaccine manufacturers, sharing the key technology for the production of pandemic influenza vaccine obtained from research since 2004, and undertaking production together.
A single batch of vaccine production involves various steps including virus inoculation, virus culture, virus inactivation, purification, preparation, packaging and batch approval.
In order to achieve protection effects while saving antigens, Sinovac Beijing used adjuvant vaccine production process.